Fluorescent Microspheres: Internal vs. External Labeling
Posted: July 19, 2019
Continuing our discussion of the different types of microspheres that can be advantageous for flow cytometry, we think it is useful to now discuss the different ways beads may be labeled with fluorophore and the typical characteristics that different dyeing strategies yield. 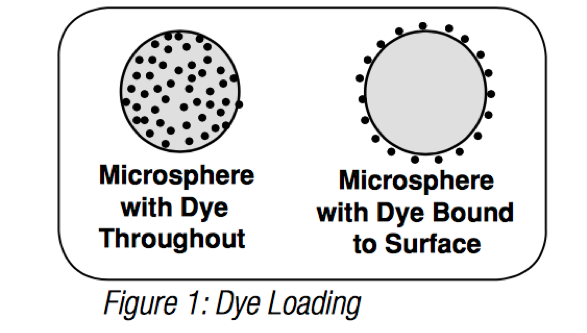
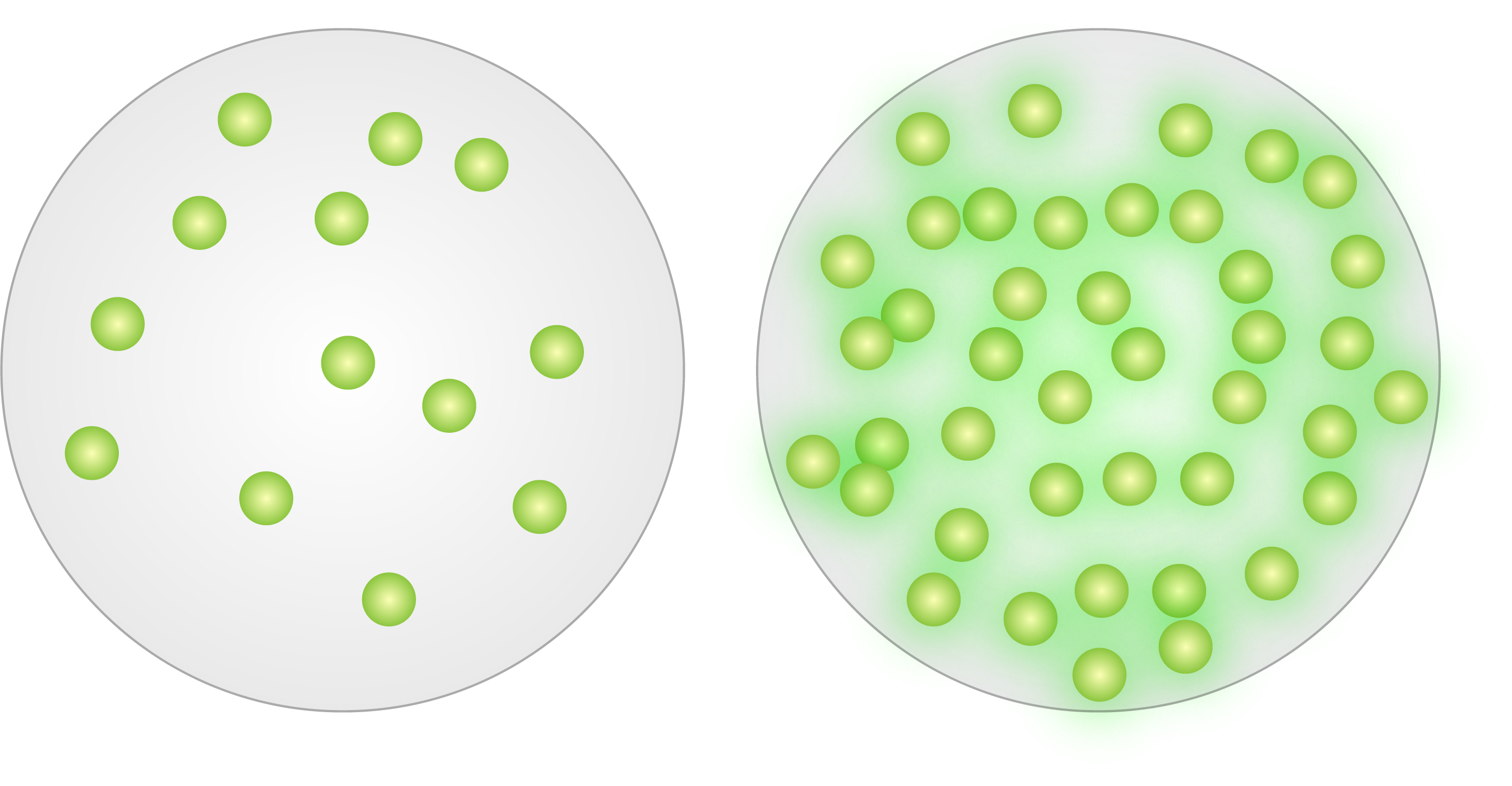
Microspheres are commonly dyed after bead synthesis, through dye entrapment (internal labeling) or surface attachment (external labeling).
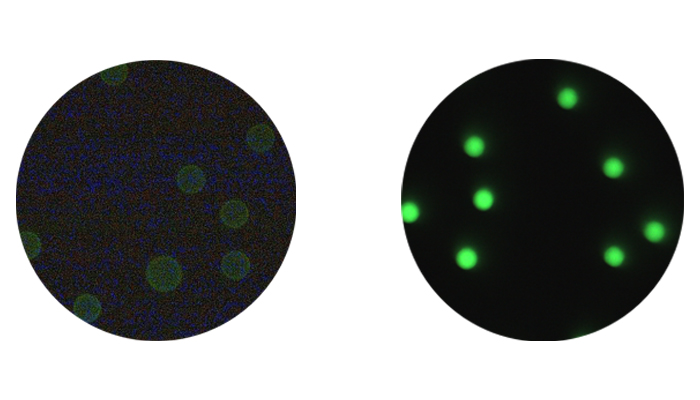
Internal labeling (or dye diffusion/entrapment) involves the swelling of polymer microspheres in an organic solvent/dye solution. The water-insolubledye diffuses into the polymer matrix and is entrapped when the solvent is removed from the microspheres (through evaporation or transfer to an aqueous phase). The great majority of our general purpose fluorescent microspheres are internally dyed, which affords many benefits, including availability of surface groups for coupling reactions, photostability and availability of a large selection of dyes. Internal dyeing generally permits a high level of dye loading (up to ~1% of the bead’s weight in dye [more might lead to self-quenching]), resulting in extremely bright microspheres. Internal dyeing is also utilized for many instrument calibration/QC particles, such as Quantum QC, where bright signal and highest stability are required.
Microspheres may also be dyed through external labeling, i.e. by attaching fluorophore to the surface. Many of our dedicated flow cytometry products feature surface-attached fluorophore, as this method yields beads that are highly suited to this demanding application. Surface labeled beads feature the same fluorophores that are used to label biologic samples, and thus exhibit environmental responsiveness (consider pH-responsiveness of FITC), spectral retention and fluorescence intensities that are in the range of labeled biologic samples. Thus, surface-labeled fluorescent microspheres are excellent surrogates for labeled cells and are exceptional calibrators for cellular expression studies requiring fluorescence quantitation.