Flow Cytometer as a QC Tool for Assessing Bead Coating Efficacy
Posted: August 12, 2019
Attaching ligands to bead surfaces is a valuable technique for the development of a variety of assays and flow applications. While there are several means of ligand attachment, covalent coupling is often employed for the immobilization of biomolecules when a very active and stable microsphere reagent is required. Covalent attachment imparts the following characteristics:
- Biomolecules are permanently bound, and will not desorb/leach over time
- Elimination of “crosstalk” between microspheres permits multiplexed tests and assays
- Ligands are favorably presented on the surface of the bead such that binding moieties are available for interaction with target molecules
Since the success of the assay relies so much on bead coating, it is important to have effective strategies for monitoring the conjugation. Traditional indirect strategies such as the Lowery or Bradford assay measure protein coating efficiency by examining the remaining protein in the supernatant after conjugation; and while these methods are effective, they can consume an expensive protein in the process.
As an alternative, the Flow cytometry can be a useful tool when assessing the efficacy of a microsphere coating procedure. 1Woods et al. discusses a flow cytometric method for coating quality assessment. They use a fluorometer to attain Relative Quantum Efficiency (Qr) of solubilized fluorophore and a flow cytometer to assess the intensity of the beads with attached Ab and having a conjugated fluorochrome. The authors note that the procedure makes 3 primary assumptions:
- It is important that only monovalent interactions occur, or the number of bound proteins will not reflect the number of surface-conjugated proteins.
- This approach assumes 100% accessibility and protein activity. If either of these parameters is <100%, this will result in an underestimation of the amount of protein on the surface using this method.
- Any proximity quenching by the fluorescently labeled ligand will again result in an underestimation of the amount of protein on the surface using this method.
Similarly, to the above study, 2Santiago et al. used PE conjugated antibodies along with flow cytometry to evaluate the degree of IgG covalent binding on the bead surface. He goes on to discuss how Parameters in the dataset were evaluated to determine whether the quality of the coating was acceptable for downstream protocols and are as follows:
- Mean: confers the intensity of the sign detected by the photomultiplier and for this reason can express the brightness of the fluorochrome
- CV: can be used to express the homogeneity of the staining as narrow peaks presents lower CVs
- Percentage: represents the number of positive cells or particles for the target molecule and can also be used to evaluate stability.
Below we have some data from our lab illustrating a homogenous and heterogeneous bead coating. You can see there is good homogeneity on the first bead as it has a narrow-defined peak as opposed to the second peak having a broader peak and more heterogeneity. Whatever your coating strategy, an established QC method will help in creating a quality end-product and utilizing flow cytometry in this process will both help ensure quality and diminish expensive consumption of your ligand.
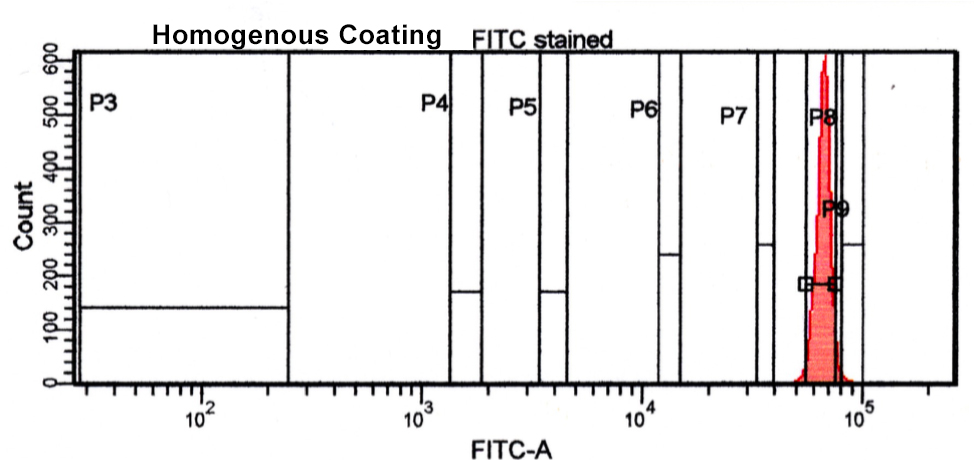
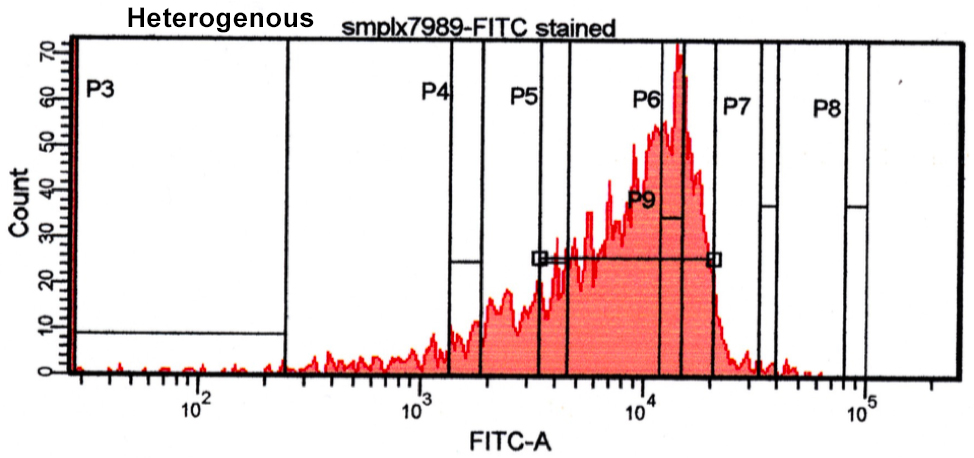
2. Santiago, M. D., Bruna De Paula Fonseca E Fonseca, Christiane De Fátima Da Silva Marques, Silva, E. D., Bertho, A. L., & Ana Cristina Martins De Almeida Nogueira. (2016). Flow Cytometry as a Tool for Quality Control of Fluorescent Conjugates Used in Immunoassays. Plos One, 11(12). doi:10.1371/journal.pone.0167669
